Folks,
I have a board I’m restoring, but this is a more general topic, so starting a new thread.
The board in question is. Vector Graphic disk controller board, and the condition is somewhat of a mystery. The rest of the system shows normal aging, and is in good condition. This board is the exception to the rest of the system, but has always been part of the system and stored with the rest of it.
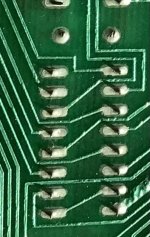
The board shows heavy oxidation on the soldered joints, and the IC pins are also oxidized. Overall the board looks ‘dirty’ on the electrical parts. The tops of the IC’s and caps, resistors look okay. At first, I thought the board was filthy with dust, so I scrubbed it gently with isopropyl alcohol and dried it. It looked exaxtly the same after this washing.
The oxidation on the solder joints is both electrically and heat resistant. To get a good electrical contact, I need to scrape the pins or joints with an exacto blade. If I want to reflow the solder joints, I have to use much higher temps on the solder station, and even if I get the joints back to normal shiny-ness with good 60/40 solder, there is a nasty residue, that needs to be scraped before it will come off.
I have already replaced a couple of IC’s on this board that were faulty. These were 74ls221’s that one half of the IC failed, but the other half functioned. Don’t know if this is related to the overall condition, but the oxidation makes troubleshooting harders and IC replacement a pain.
So, Ive seen bad boards before from wet environments, or from rodents nesting in cases, but this is a mystery.
First question: is there anything that might dissolve the oxidation without eating up the solder joints or causing further damage?
Second question: has anyone seen similar damage?
Third question: Could the IC damage be related?
Fourth question: what might have caused this, in a system that shows no other damage like this?
I will try to post some pictures to illustrate what this looks like.
I have a board I’m restoring, but this is a more general topic, so starting a new thread.
The board in question is. Vector Graphic disk controller board, and the condition is somewhat of a mystery. The rest of the system shows normal aging, and is in good condition. This board is the exception to the rest of the system, but has always been part of the system and stored with the rest of it.
The board shows heavy oxidation on the soldered joints, and the IC pins are also oxidized. Overall the board looks ‘dirty’ on the electrical parts. The tops of the IC’s and caps, resistors look okay. At first, I thought the board was filthy with dust, so I scrubbed it gently with isopropyl alcohol and dried it. It looked exaxtly the same after this washing.
The oxidation on the solder joints is both electrically and heat resistant. To get a good electrical contact, I need to scrape the pins or joints with an exacto blade. If I want to reflow the solder joints, I have to use much higher temps on the solder station, and even if I get the joints back to normal shiny-ness with good 60/40 solder, there is a nasty residue, that needs to be scraped before it will come off.
I have already replaced a couple of IC’s on this board that were faulty. These were 74ls221’s that one half of the IC failed, but the other half functioned. Don’t know if this is related to the overall condition, but the oxidation makes troubleshooting harders and IC replacement a pain.
So, Ive seen bad boards before from wet environments, or from rodents nesting in cases, but this is a mystery.
First question: is there anything that might dissolve the oxidation without eating up the solder joints or causing further damage?
Second question: has anyone seen similar damage?
Third question: Could the IC damage be related?
Fourth question: what might have caused this, in a system that shows no other damage like this?
I will try to post some pictures to illustrate what this looks like.